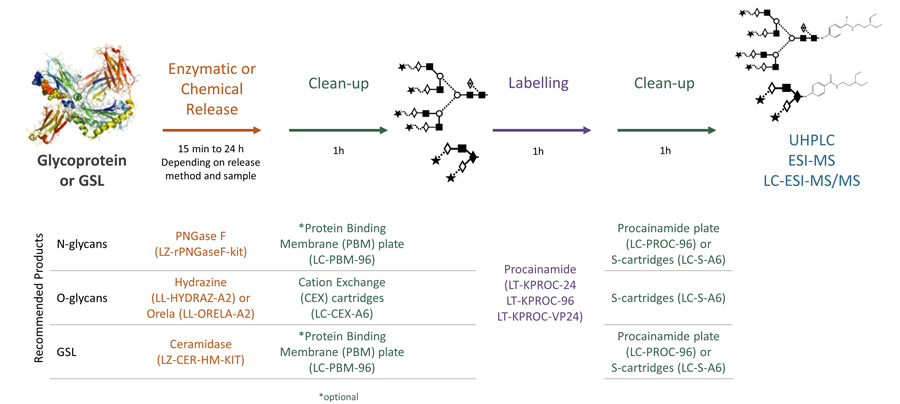
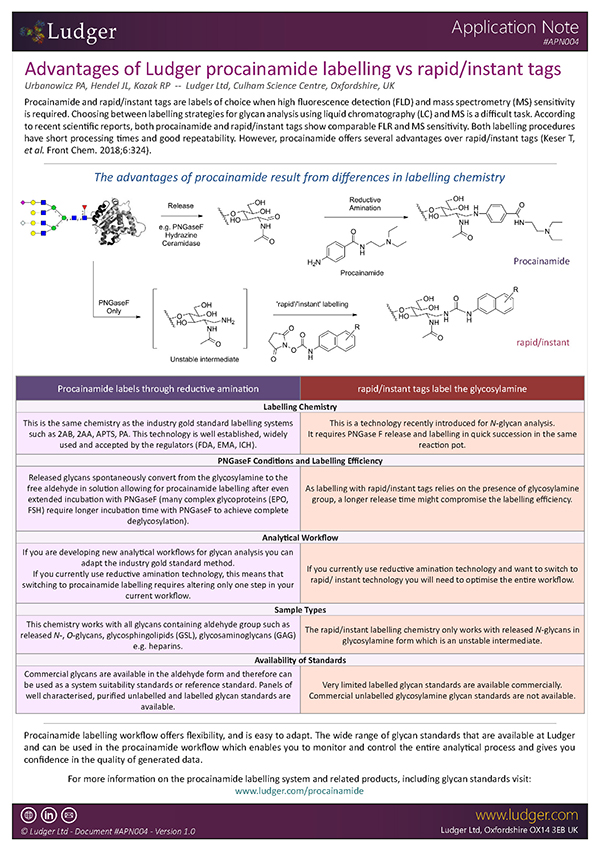
Procainamide labelling: A reliable and flexible approach to glycan analysis
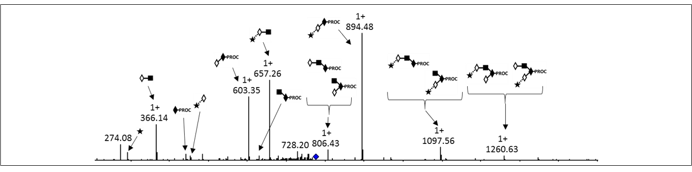
Procainamide labelling permits glycan identification by either mass spectrometry or (U)HPLC, and because of its improved ionisation efficiency compared to 2AB labelling it can permit identification of minor glycans (<1% relative peak area) by ESI-MS.
Ludger's procainamide labelling system is suitable for N-glycans, O-glycans, GSL-glycans, heparin or any sugar with a reducing terminus. Also, once labelled with procainamide the glycans can be incubated with exoglycosidases.
As well as labelling kits we offer a clean up system for use after labelling and a range of procainamide labelled glycan standards (including an N-glycan library and glucose homopolymer ladder). Using products together (including LudgerZyme PNGaseF, LZ-rPNGaseF-kit), N-glycan sample preparation can be carried out in one day (for 96 samples)