Glycan Analysis Services - Level 2
This module is an extension of the Level 1 HILIC profiling work to gain more detailed information on the glycan structures and their relative proportions. This can be achieved by Exoglycosidase digestion followed by HILIC-UPLC and/or by HILIC-UPLC-FLR-ESI-MS/MS analyses.
Level 2 Exoglycosidase digestion followed by HILIC-UPLC
Exoglycosidases are the enzymes that cleave the glycosidic linkage of a terminal monosaccharide from glycan. By using positionally specific exoglycosidases in a customised enzyme array, the removed glycan residues can be identified by monosaccharide type, linkage, and sequence. Because each type of monosaccharide is removed separately a series or a mixture of exoglycosidases is required to identify the glycan structure.
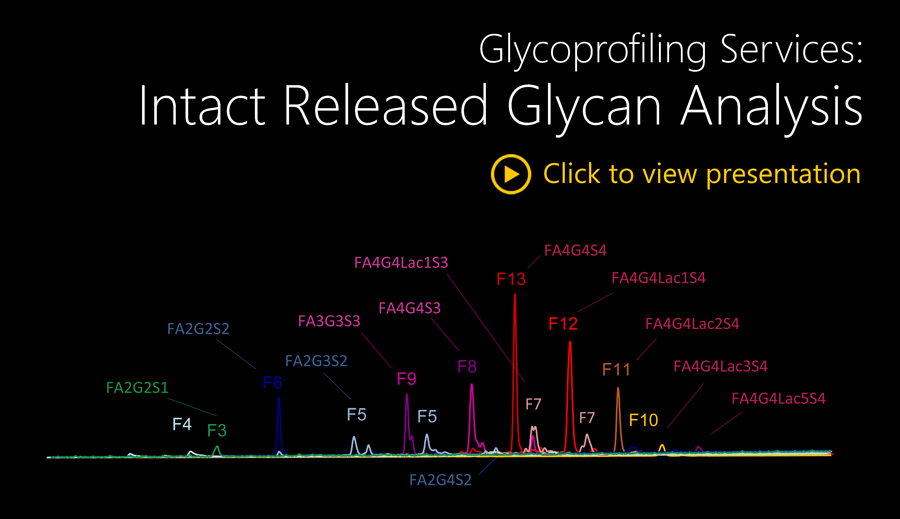
This module provides HILIC-UPLC N- and/or O-glycan profiles before and after specific exoglycosidase digestions together with glycan structures and their relative quantitation. The analysis will be performed on single or triplicate N- and/or O-glycan samples, released and fluorescently labelled under the Level 1 HILIC profiling, alongside Ludger positive and negative controls and system suitability standards.
This module is suitable for:
- glycan assignment (monosaccharide type, order, linkage)
- quality control - profile comparisons to monitor known structures
- monitoring batch to batch consistency
- comparability studies
In order to gain more detailed information and increase confidence in the glycan structures assignment and their relative proportions additional Level 2 HILIC-UPLC-FLR-ESI-MS/MS is recommended.
Sample types:
Released and fluorescently labelled N- and/or O-glycans from:
- Biopharmaceuticals: mAbs, glycoprotein hormones (e.g. follicle stimulating hormone (FSH) and erythropoietin (EPO), Fc fusion proteins, vaccines)
- Cells: mammalian cell lines, bacterial cell components
- Biological fluids, tissues and others
- Glycoproteins set in SDS-gel (N-glycans only)
- COVID-19 patient samples (e.g. plasma, tissues)
- SARS-CoV-2 infected cell lines
Workflow for Level 2 Exoglycosidase digestion followed by HILIC-UPLC
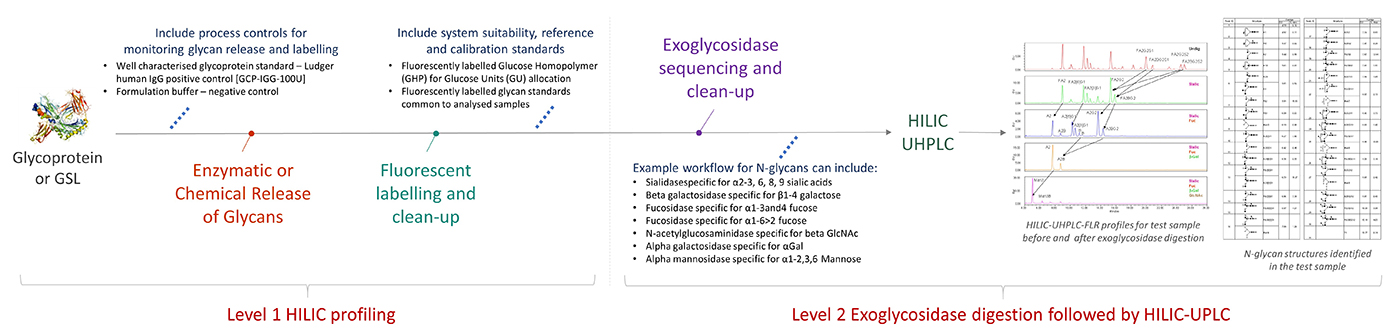
click to enlarge 
Released and fluorescently labelled glycans (Level 1 HILIC profiling) are digested with a series or a mixture of specific exoglycosidases purified and analysed by HILIC-HPLC or HILIC-UHPLC.
Report
Final report contains:
- HILIC-UPLC profiles for system suitability standards and Ludger positive controls before and after exoglycosidase digestions
- HILIC-UPLC profiles for client samples and buffer negative controls before and after exoglycosidase digestions
- Glucose unit (GU) values
- Glycan structures (including monosaccharide type, order and linkage) and their relative proportions
- Summary of findings (e.g. G0:G1:G2; % high mannose; % fucosylation; % Galα1-3Gal)
Module Level 2 Exoglycosidase digestion followed by HILIC-UPLC has:
2 options for N-glycans characterisation to choose between:
- G-L2N-Ex - Option 1: Exoglycosidase sequencing with HILIC-UPLC profiling on a single released aliquot of each sample
- G-L2N-Ex-t - Option 2: Exoglycosidase sequencing with HILIC-UPLC profiling on aliquots of triplicate releases of each sample
2 options for O-glycans characterisation to choose between:
- G-L2O-Ex - Option 1: Exoglycosidase sequencing with HILIC-UPLC profiling on a single released aliquot of each sample
- G-L2O-Ex-t - Option 2: Exoglycosidase sequencing with HILIC-UPLC profiling on aliquots of triplicate releases of each sample
Relevant Application Note
Relevant Posters

Kozak RP, Kotsias M, Hendel JL, Urbanowicz PA
Presented at: WCB 2019
Reston, USA. Nov 11-13th 2019

Urbanowicz PA, Hendel JL, Kotsias M, Kozak RP
Presented at: BioProduction 2019
Frankfurt, Germany. Nov 5-7th 2019

Kozak RP, Royle L, Liew LP, Spencer DI, Fernandes DL
Presented at WCBP 2016: 20th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products
Washington DC, United States. January 2016
Keywords: Gal alpha 1-3 gal, IgG, monoclonal antibody (mAb), glycosylation critical quality attribute (GCQA), QbD, procainamide, UHPLC, ESI-MS/MS






