Glycan Analysis Services - Level 3
This module is an extension of the Level 1 and Level 2 N-glycan characterisation work to gain more detailed information on the individual N-glycan sites. This can be achieved by detailed site-specific glycosylation analysis and N-glycan site % occupancy analysis.
Level 3 N-glycan Site Occupancy Analysis
The number of potential N-glycosylation sites is determined by the amino acid sequence of the glycoprotein. This module determines which N-glycan sites are occupied or unoccupied by glycans by measuring the incorporation of 18O from 18O water during the removal of N-glycans by PNGase F. This adds a mass of 3Da to any peptide which has an N-glycan site occupied. These peptides are then analysed by C18-LC-MS/MS or MALDI-TOF-MS/MS to obtain the relative proportions of the N-glycan-containing peptides with and without the additional 3Da mass.
Determination of N-glycan site occupancy is a non-standard glycoprofiling module because the exact conditions required will differ for each glycoprotein analysed.
The amino acid sequence and the formulation buffer can both have an effect on the exact protocol followed as well as on generated data.
The analysis will be performed on a single glycoprotein sample, alongside Ludger positive and negative controls, and system suitability standards.
This module is suitable for:
- quality control – % occupancy comparison
- monitoring batch to batch consistency
- comparability studies
In order to gain more detailed information on N-glycan composition at a specific glycosylation site, Level 3 site-specific glycosylation analysis is recommended.
Sample types:
Glycoprotein and glycopeptide samples containing at least one N-glycosylation site:
- Biopharmaceuticals: mAbs, glycoprotein hormones (e.g. follicle stimulating hormone (FSH) and erythropoietin (EPO), Fc fusion proteins, vaccines)
- Glycoproteins purified from cells and cell components
- Glycoproteins purified from biological fluids, tissues and others
- COVID-19 patient samples (e.g. plasma, tissues)
- SARS-CoV-2 infected cell lines
Workflow for Level 3 N-glycan Site Occupancy Analysis
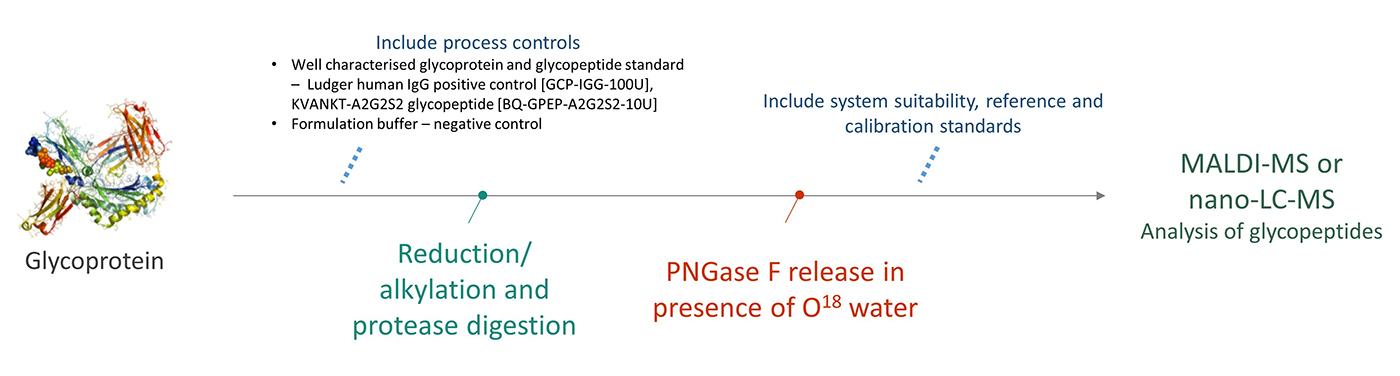
click to enlarge 
Report
Final report contains:
- C18-LC-MS/MS or MALDI-TOF-MS/MS spectra for system suitability standards and Ludger positive and negative controls
- C18-LC-MS/MS or MALDI-TOF-MS/MS spectra for peptides de-glycosylated in the presence of 18O water for client samples
- Proportion of N-glycan occupancy at each glycosylation site
- Summary of findings