Glycan Analysis Services - Level 2
This module is an extension of the Level 1 HILIC profiling work to gain more detailed information on the glycan structures and their relative proportions. This can be achieved by Exoglycosidase digestion followed by HILIC-UPLC and/or by HILIC-UPLC-FLR-ESI-MS/MS analyses.
Level 2 HILIC-UHPLC-FLR-ESI-MS/MS
A combination of the hydrophilic interaction ultrahigh performance liquid chromatography (HILIC-UPLC) and electrospray ionization mass spectrometry (ESI–MS) can greatly improve glycan analysis by providing specific structural information.
The stoichiometric attachment of one label per glycan allows the relative quantitation of different glycan species based on fluorescence (FLR) and the ESI-MS and MS/MS fragmentation data can provide composition and sequence data for each glycan.
This module is an extension of the Level 1 HILIC profiling work to gain more detailed information on the glycan structures and their relative proportions. This module provides HILIC-UHPLC-FLR profiles, ESI-MS/MS spectra, mass composition and sequence data matching glucose unit (GU) values, m/z and MS/MS sequencing data. The analysis will be performed on single or triplicate N- and/or O-glycan samples, released and fluorescently labelled under the Level 1 HILIC profiling module, alongside Ludger positive and negative controls, and system suitability standards.
This module is suitable for:
- glycan assignment based on mass composition sequence data
- quality control - profile comparisons to monitor known structures
- monitoring batch to batch consistency
- comparability studies
In order to gain more detailed information (such as monosaccharide type and linkage) and increase confidence in the glycan structures assignments and their relative proportions Level 2 Exoglycosidase digestion followed by HILIC-UPLC is recommended.
Sample types:
Released and fluorescently labelled N- and/or O-glycans released from:
- Biopharmaceuticals: mAbs, glycoprotein hormones (e.g. follicle stimulating hormone (FSH) and erythropoietin (EPO), Fc fusion proteins, vaccines)
- Cells: mammalian cell lines, bacterial cell components
- Biological fluids, tissues and others
- Glycoproteins set in SDS-gel (N-glycans only)
- COVID-19 patient samples (e.g. plasma, tissues)
- SARS-CoV-2 infected cell lines
Workflow for Level 2 HILIC-UPLC-FLR-ESI-MS/MS
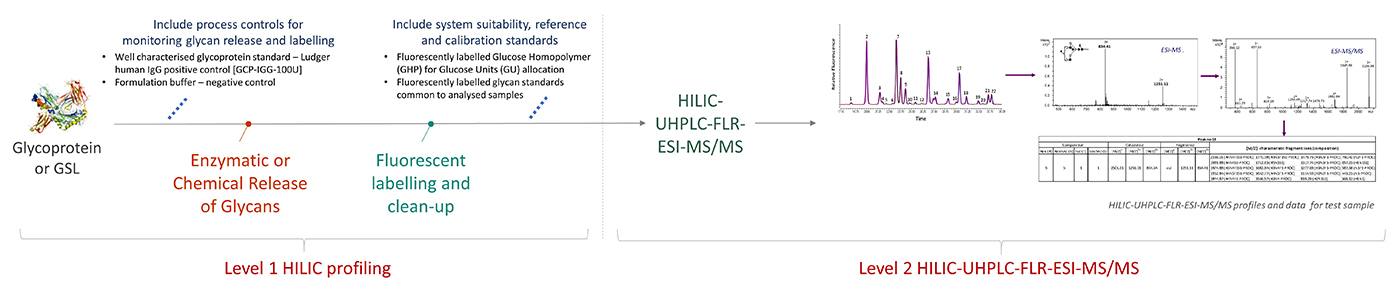
click to enlarge 
Released and fluorescently labelled glycans (Level 1 HILIC profiling) are analysed by HILIC-UHPLC-FLR-ESI-MS/MS.
Report
Final report contains:
- HILIC-UPLC profiles for system suitability standards and Ludger positive controls
- HILIC-UPLC profiles for client samples and buffer negative controls
- Glucose unit (GU) values
- Mass composition data matching GU, m/z and MS/MS sequencing data
- Proposed glycan structures and their relative proportions
- Summary of findings (e.g. G0:G1:G2; % high mannose; % fucosylation)
Module Level 2 HILIC-UPLC-FLR-ESI-MS/MS has:
2 options for N-glycans characterisation to choose between:
- G-L2-N-MSMS - Option 1: HILIC-UPLC-FLR-ESI-MS/MS analysis of the fluorescent labelled glycans- single sample analysis
- G-L2-N-MSMS-t - Option 2: HILIC-UPLC-FLR-ESI-MS/MS analysis of the fluorescent labelled glycans- triplicate samples analysis
2 options for O-glycans characterisation to choose between:
- G-L2-O-MSMS - Option 1: HILIC-UPLC-FLR-ESI-MS/MS analysis of the fluorescent labelled glycans- single sample analysis
- G-L2-O-MSMS-t - Option 2: HILIC-UPLC-FLR-ESI-MS/MS analysis of the fluorescent labelled glycans- triplicate samples analysis
Relevant Application Note
Relevant Posters

Kozak RP, Kotsias M, Hendel JL, Urbanowicz PA
Presented at: WCB 2019
Reston, USA. Nov 11-13th 2019

Urbanowicz PA, Hendel JL, Kotsias M, Kozak RP
Presented at: BioProduction 2019
Frankfurt, Germany. Nov 5-7th 2019

Thomson RI, Gardner RA, Kozak RP, Smith J, Strohfeldt K, Osborn HMI, Spencer DI
Presented at:WCBP 2018: 22nd Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products
Washington D.C., United States. January 30-February 1st 2018

Wongtrakul-Kish K, Kozak RP, Spencer D
Presented at the GlycoPar Symposium & Workshop
Liverpool, UK. September 12-14th 2016
Keywords: Procainamide, LC-MS, HILIC-HPLC-ESI-MS/MS, parasitic glycans, insect vector glycans, host glycans, GlycoPar

Kozak RP, Royle L, Liew LP, Spencer DI, Fernandes DL
Presented at WCBP 2016: 20th Symposium on the Interface of Regulatory and Analytical Sciences for Biotechnology Health Products
Washington DC, United States. January 2016
Keywords: Gal alpha 1-3 gal, IgG, monoclonal antibody (mAb), glycosylation critical quality attribute (GCQA), QbD, procainamide, UHPLC, ESI-MS/MS








