Glycan Analysis Services - Level 1
MALDI profiling
A matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS) of released and permethylated N- and O-glycans is a rapid and well known approach for glycan analysis. Permethylation is the most popular derivatization of carbohydrates for MS detection, as it improves and enhances ionization efficiency of glycans, stabilizes the labile sialic acid moieties, and enables the detection of both neutral and acidic glycans in positive ion mode. Level 1 MALDI profiling determines the overall N- and O-glycan profiles.
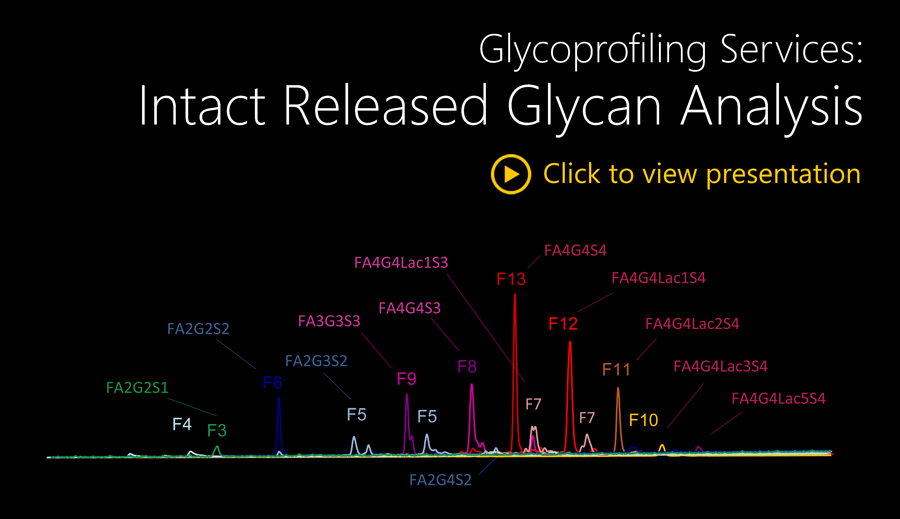
This module provides MALDI-TOF-MS N- and/or O-glycan spectra, and m/z data for mass composition matching of the charged and neutral glycans. The analysis will be performed on a triplicate release of samples (typically 5-100 µg for each release), an equivalent amount (by volume) of sample buffer negative controls, alongside Ludger positive and negative controls and system suitability standards.
This module is suitable for:
- preliminary glycan assignment
- quality control - profile comparisons to monitor known structures
- monitoring batch to batch consistency
- comparability studies
Sample types:
- Biopharmaceuticals: mAbs, glycoprotein hormones (e.g. follicle stimulating hormone (FSH) and erythropoietin (EPO), Fc fusion proteins, vaccines).
- Cells: mammalian cell lines, bacterial cell components
- Biological fluids, tissues and others
- Glycoproteins set in SDS-gel (N-glycans only)
- COVID-19 patient samples (e.g. plasma, tissues)
- SARS-CoV-2 infected cell lines
Workflow for Level 1 MALDI profiling
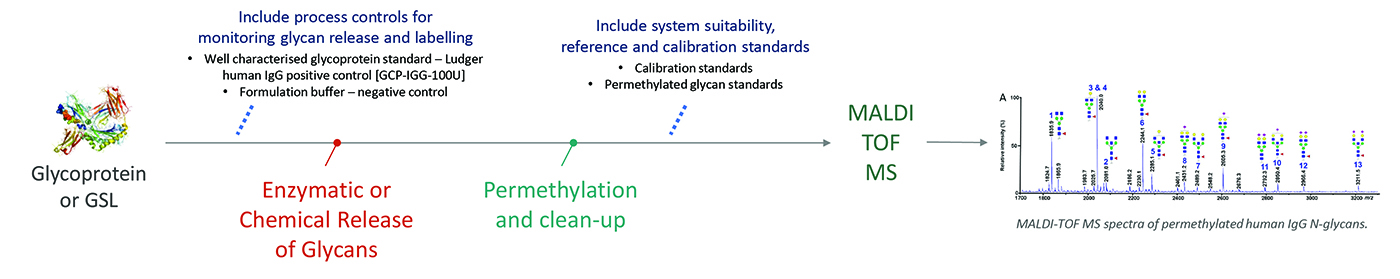
N-glycans are released from glycoprotein by digestion with PNGAse F or PNGAse A.
O-glycans are released from glycoprotein by hydrazinolysis or Orela reagent.
Released glycans are permethylated, purified and analysed by MALDI-TOF-MS.
Permethylation is the process of derivatizing all the hydroxyl and N-acetyl groups by the addition of a methyl group. Permethylation also methyl esterifies the carboxy function on the sialic acid. Both these derivatizations lead to a stabilization of the sialic acids and to a significant enhancement in the MS analysis.
Report
Final report contains:
- MALDI-TOF-MS spectra for system suitability standards and Ludger positive controls
- MALDI-TOF-MS spectra for client samples and buffer negative controls
- m/z data for mass composition matching of the charged and neutral glycans
- Relative proportions of detected peaks
- Summary of findings
Module Level 1: MALDI profiling has 2 options to choose between:
- G-L1N-MALDI: N-glycoprofiling on triplicate sample releases + formulation buffer + Ludger controls.
- G-L1O-MALDI: O-glycoprofiling on triplicate sample releases + formulation buffer + Ludger controls.
Relevant Posters

Kotsias M, Gardner R, Kozak RP, Wuhrer M, Spencer D
Presented at:12th Jenner Glycobiology and Medicine Symposium: Translational Glycobiology
Dubrovnik, Croatia. May 6-9th 2017
Keywords: Colorectal cancer (CRC), O-glycosylation, permethylation, MALDI-TOF-MS, high throughput analysis, GlycoCan

Shubhakar A, Spencer D, Wuhrer M, Fernandes DL
Presented at the 38th Annual Congress of Pharmacology Society of Chile
Castro, Chile. November 26-29th 2016
Keywords: Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF-MS), permethylation, high throughput (HTP), N-glycans, O-glycans

Shubhakar A, Kozak RP, Badía Tortosa C, Gardner RA, Royle L, Wuhrer M, Spencer DI, Fernandes DL
Presented at PEGS 2014: Glycosylation Analysis for Biopharmaceuticals Workshop
Boston, United States. May 2014
Keywords: high throughput, permethylation, V-Tag, glycosylation critical quality attribute (GCQA), IgG, erythropeitin (EPO), UHPLC, MALDI-MS




