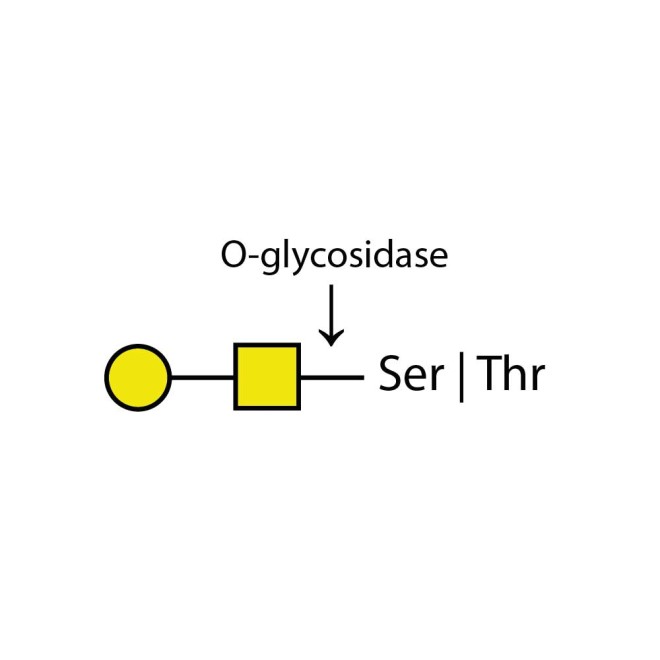
O-glycosidase
References:
1. Bhavanandan, V.P., J. Umemoto and E.A. Davidson. Characterization of an endo-alpha-N-acetylgalactosaminidase from Diplococcus pneumoniae. Biochem Biophys 70: 738-74 5 (1976 ).
2. Fan, J.Q., K. Yamamoto, H. Kumagai and T. Tochikura. Induction and efficient purification of endo-alpha-N-acetyl- D-galactosaminidase from Alcaligenes sp. Agric Biol Chem 54: 233-23 4 (1990 ).
3. Glasgow, L R., J.C. Paulson and R.L. Hill. Systematic purification of five glycosidases from Streptococcus pneumoniae. J Biol Chem 252: 8615-8 623 (1977).
4. Iwase, H. and K . Hotta. Release of O-linked glycoprotein glycans by endo-alpha-N-acetyl-D-galactosaminidase. Meth Mol Biol 14: 151-159 (1993).
5. Unemoto, J., V.P. Bhavanandan and E.A. Davidson. Purification and properties of an endo-alpha-N-acetyl-D-galactosaminidase from Diplococcus pneumoniae. J Biol Chem 252: 8609-8 614 (1977).
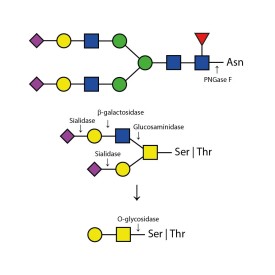
O-glycosidase cleaves only unsubstituted Gal-β(1-3)GalNAc-α disaccharides attached to the serine or threonine residues of glycoproteins or glycopeptides.
Substitutions such as sialic acid, galactose, fucose or N-acetylglucosamine must first be removed with the appropriate exoglycosidase before treatment with Endo-O-Glycosidase. At minimum, a sialadase such as Sialadase Au α(2-3,6,8,9), part number E-S001, is almost always required to remove sialic acids.
Kit includes enzyme plus reaction buffer. Sufficient for up to 60 reactions.
Product Specifications:
Specificity:
O-glycosidase leaves only unsubstituted Gal-β(1-3)GalNAc-α disaccharides attached to the serine or threonine residues of glycoproteins or glycopeptides.
Substitutions such as sialic acid, galactose, fucose or N-acetylglucosamine must first be removed with the appropriate exoglycosidase prior to treatment with Endo-O-Glycosidase. At minimum, a sialadase such as Sialadase Au α(2-3,6,8,9), part number E-S001, is almost always required to remove sialic acids.
Source: Recombinant Streptococcus pneumoniae in E.Coli
EC: 3.2.1.97
Contents:
60 µL aliquot of enzyme (75 mU) in 50 mM sodium phosphate, pH 7.5.
5x Reaction Buffer 5.0 (250 mM sodium phosphate, pH 5.0)
Specific Activity: >12 U/mg
Activity: >1.25 U/mL
Molecular weight: 180,000 daltons
pH range: 5-7, optimum 5.0
Suggested usage:
1. Add up to 100 µg of glycoprotein to a tube.
2. Add de-ionized water to a total of 13 µL.
3. Add 4 µL of 5x Reaction Buffer 5.0.
4. Add 1 µL of Sialadase Au α(2-3,6,8,9), part number E-S001.
5. Add 2 µL of O-Glycosidase.
6. Incubate for 1 hour at 37°C.
Specific Activity: Defined as the amount of enzyme required to produce 1 µmole of p-nitrophenol (pNP) in 1 minute at 37°C, pH 5.0 from p-nitrophenyl-2-acetamido-2-deoxy-3-O-(β-D-galactopyranosyl)-α-D-galactopyranoside.
Storage: Store enzyme at 4°C.
Stability: Stable at least 12 months when stored properly. Several days exposure to ambient temperature will not reduce activity. Active for at lease 5 days under reaction conditions.
Purity: O-Glycosidase is tested for contaminating protease as follows; 10 µg of denatured BSA is incubated for 24 hours at 37°C with 2 µL of enzyme. SDS-PAGE analysis of the treated BSA shows no evidence of degradation.