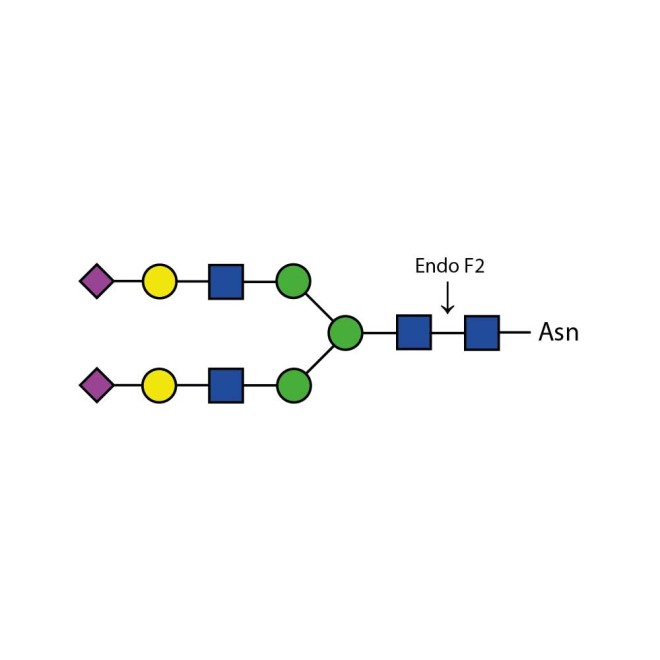
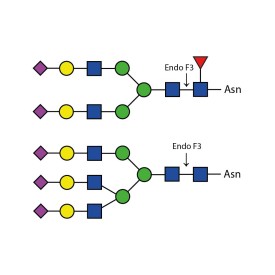
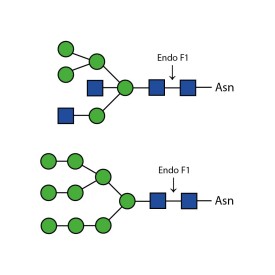
Endoglycosidase F2
References:
1. Maley P., R. B. Trimble, A. L. Tarentino and T. H. Plummer Jr. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem 180:195-204 (1989).
2. Plummer, T. H. Jr, A. W. Phelan and A. L. Tarentino. Porcine fibrinogen glycopeptides: substrates for detecting endo-N-acetylglucosaminidases F2 and F3. Anal Biochem 235:98-101 (1996).
3. Reddy A., B. G. Grimwood, T. H. Plummer Jr and A. L. Tarentino. High- level expression of the Endo-beta-N- acetylglucosaminidase F2 gene in E.coli: one step purification to homogeneity. Glycobiology 8:633-636 (1998).
4. Tarentino, A. L., C. M. Gomez and T. H. Plummer Jr. Deglycosylation of Asparagine-Linked Glycans by Peptide:N-Glycosidase F. Biochemistry 24:4665-4671 (1985).
5. Tarentino A. L., G. Quinones, W. P. Schrader, L. M. Changchien and T. H. Plummer Jr. Multiple endoglycosidase (Endo) F activities expressed by Flavobacterium meningosepticum. Endo F1: molecular cloning, primary sequence, and structural relationship to Endo H. J Biol Chem 267:3868-3872 (1992).
6. Tarentino A. L., G. Quinones, L. M. Changchien, and T. H. Plummer Jr. Multiple endoglycosidase F activities expressed by Flavobacterium meningosepticum endoglycosidases F2 and F3: Molecular cloning, primary sequence, and enzyme expression. J Biol Chem 268(13):9702-9708 (1993).
7. Tarentino A. L. and T. H. Plummer Jr. Substrate specificity of Flavobacterium meningosepticum: Endo F2 and endo F3: purity is the name of the game. Glycobiology 4:771-773 (1994).
8. Tarentino, A. L. and T. H. Plummer Jr. Enzymatic deglycosylation of asparagine- linked glycans: purification, properties and specificity of oligosaccharide- cleaving enzymes from Flavobacterium meningosepticum. Methods in Enzymology 230:44-57 (1994).
9. Tarentino A. L., G. Quinones and T. H. Plummer Jr. Overexpression and purification of non-glycosylated recombinant endo-beta-N- acetylglucosaminidase F3. Glycobiology 5:599-601 (1995).
10. Trimble, R. B. and A. L. Tarentino. Identification of Distinct Endoglycosidase (Endo) Activities in Flavobacterium meningosepticum: Endo F1, Endo F2 and Endo F3. J. Biol Chem 266:1646-1651 (1991).
Endo F2 cleaves N-linked (asparagine-linked) biantennary oligosaccharides from glycoproteins. It also will cleave high mannose glycans but at a 40x reduced rate. It cleaves between the two N-acetylglucosamine residues in the diacetyl chitobiose core of the oligosaccharide, generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine. In contrast, PNGase F removes the oligosaccharide intact.
Endoglycosidase F2 is less sensitive to protein conformation than PNGase F and is, therefore, more suitable for deglycosylation of native proteins. However, for optimal results, denaturation of the glycoprotein is recommended.
Recombinant gene from Elizabethkingia miricola in E. Coli
Kit includes enzyme plus reaction buffer. Sufficient for up to 60 reactions.
Product Specification
Source: Recombinant Elizabethkingia miricola in E. Coli
EC: 3.2.1.96
Alternative Names: Endo F2, Endoglycosidase F2, endo-β-N-acetylglucosaminidase F2
Specificity:
Cleaves all asparagine-linked biantennary oligosaccharides and high mannose (though at a 40X reduced rate) N-glycans from peptides and proteins
Contents:
60 µL aliquot of enzyme (0.3 U) in 10 mM sodium acetate 25mM NaCl, pH 4.5
5x Reaction Buffer - 250 mM sodium acetate, pH 4.5
Specific Activity: >20 U/mg
Activity: >5 U/mL
Molecular weight: 32,000 daltons
Suggested usage:
1. Add up to 200 µg of glycoprotein to an Eppendorf tube. Adjust to 38 µL final volume with de-ionized water.
2. Add 10 µL 5x Reaction Buffer 4.5
3. Add 2.0 µL of Endo F2. Incubate 1 hour at 37°C.
Specific Activity:
Defined as the amount of enzyme required to catalyze the release of N-linked oligosaccharides from 1 micromole of denatured porcine fibrinogen in 1 minute at 37°C, pH 5.5. Cleavage is monitored by SDS-PAGE (cleaved finbrinogen migrates faster).
Storage: Store enzyme at 4°C.
Stability: Stable at least 12 months when stored properly. Several days exposure to ambient tempertures will not reduce activity.
Purity: Endoglycosidase F2 is tested for contaminating protease as follows; 10 µg of denatured BSA is incubated for 24 hours at 37°C with 2 µL of enzyme. SDS-PAGE analysis of the treated BSA shows no evidence of degradation.
The production host strain has been extensively tested and does not produce any detectable glycosidases.