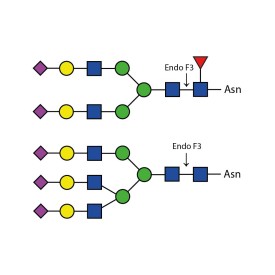
Endoglycosidase F1
References:
1. Maley P., R. B. Trimble, A. L. Tarentino and T. H. Plummer Jr. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal Biochem 180:195-204 (1989).
2. Plummer, T. H. Jr, A. W. Phelan and A. L. Tarentino. Porcine fibrinogen glycopeptides: substrates for detecting endo-N-acetylglucosaminidases F2 and F3. Anal Biochem 235:98-101 (1996).
3. Reddy A., B. G. Grimwood, T. H. Plummer Jr and A. L. Tarentino. High- level expression of the Endo-beta-N- acetylglucosaminidase F2 gene in E.coli: one step purification to homogeneity. Glycobiology 8:633-636 (1998).
4. Tarentino, A. L., C. M. Gomez and T. H. Plummer Jr. Deglycosylation of Asparagine-Linked Glycans by Peptide:N-Glycosidase F. Biochemistry 24:4665-4671 (1985).
5. Tarentino A. L., G. Quinones, W. P. Schrader, L. M. Changchien and T. H. Plummer Jr. Multiple endoglycosidase (Endo) F activities expressed by Flavobacterium meningosepticum. Endo F1: molecular cloning, primary sequence, and structural relationship to Endo H. J Biol Chem 267:3868-3872 (1992).
6. Tarentino A. L., G. Quinones, L. M. Changchien, and T. H. Plummer Jr. Multiple endoglycosidase F activities expressed by Flavobacterium meningosepticum endoglycosidases F2 and F3: Molecular cloning, primary sequence, and enzyme expression. J Biol Chem 268(13):9702-9708 (1993).
7. Tarentino A. L. and T. H. Plummer Jr. Substrate specificity of Flavobacterium meningosepticum: Endo F2 and endo F3: purity is the name of the game. Glycobiology 4:771-773 (1994).
8. Tarentino, A. L. and T. H. Plummer Jr. Enzymatic deglycosylation of asparagine- linked glycans: purification, properties and specificity of oligosaccharide- cleaving enzymes from Flavobacterium meningosepticum. Methods in Enzymology 230:44-57 (1994).
9. Tarentino A. L., G. Quinones and T. H. Plummer Jr. Overexpression and purification of non-glycosylated recombinant endo-beta-N- acetylglucosaminidase F3. Glycobiology 5:599-601 (1995).
10. Trimble, R. B. and A. L. Tarentino. Identification of Distinct Endoglycosidase (Endo) Activities in Flavobacterium meningosepticum: Endo F1, Endo F2 and Endo F3. J. Biol Chem 266:1646-1651 (1991).
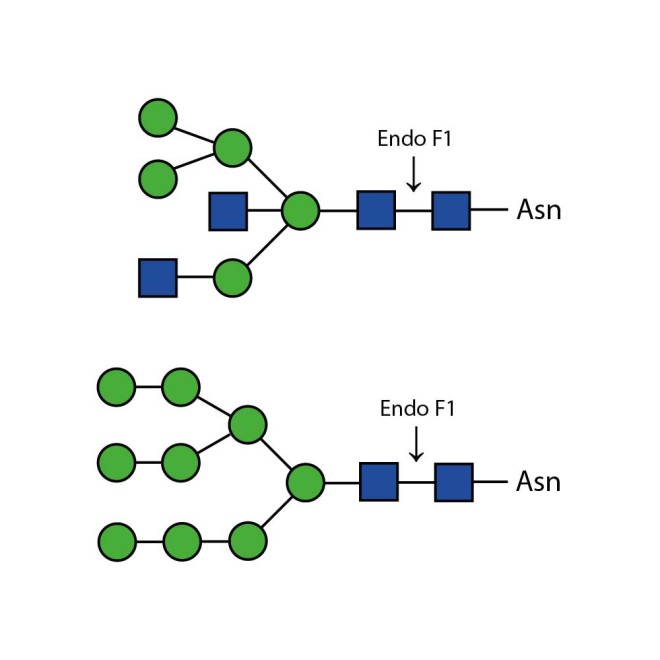
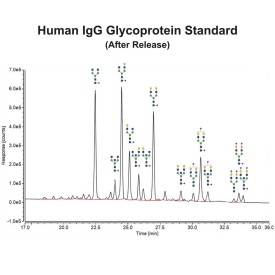
Endo F1 cleaves high mannose and some hybrid type N-glycans from peptides and proteins.
Endo F1 cleaves Asparagine-linked high mannose and some hybrid oligosaccharides. Core fucosylation reduces the activity by 50 fold. Endoglycosidase F1 will hydrolyze sulfate containing high-mannose chains. It cleaves between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide, generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine. In contrast, PNGase F removes the oligosaccharide intact.
Product Specification
Source: Recombinant Elizabethkingia miricola in E. Coli
EC: 3.2.1.96
Alternative Names: Endo F1, Endoglycosidase F1, endo-β-N-acetylglucosaminidase F
Endo F1 Specificity: Cleaves all asparagine-linked high mannose and some hybrid oligosaccharides
Contents:
60 µL aliquot of enzyme (1 U) in 20 mM Tris-HCl, pH 7.5
5x Reaction Buffer – 250 mM sodium phosphate, pH 5.5
Recommended Reagents
included with E-EF01 and E-EF01-20: 1 vial: 5x Reaction Buffer, 250 mM sodium phosphate, pH5.5
Activity ≥ 17 U/ml
Specific Activity ≥ 16 U/mg
Molecular Weight: 32 kD
Specific Activity : Defined as the amount of enzyme required to catalyze the release of N-linked oligosaccharides from 1 micromole of denatured Ribonuclease B (RNase B) in 1 minute at 37°C, pH 5.5. Cleavage is monitored by SDS-PAGE (cleaved RNase B migrates faster).
Formulation: The enzyme is provided as a sterile-filtered solution in 20mM Tris-HCl, pH 7.5
Storage: Store enzyme at 4°C. Do not freeze.
Stability: Several days exposure to ambient temperatures will not reduce activity. Stable at least 12 months when stored properly.
Specificity
Ludger Endo F1 cleaves Asparagine-linked high mannose or hybrid oligosaccharides. It cleaves between the two N-acetylglucosamine residues in the diacetylchitobiose core of the oligosaccharide, generating a truncated sugar molecule with one N-acetylglucosamine residue remaining on the asparagine. In contrast, PNGase F removes the oligosaccharide intact.
Quality & Purity
Ludger Endo F1 is tested for contaminating protease as follows: 10 μg of denatured BSA is incubated at 37°C for 24 hours with 2 μl of enzyme. SDS-PAGE analysis of the treated BSA shows no evidence of degradation. The production host strain has been extensively tested and does not produce any detectable glycosidases.
Directions for use
- Add up to 200 μg of glycoprotein to an Eppendorf tube.
- Adjust to 38 μl final volume with de-ionized water.Add 10 μl 5x Reaction Buffer 5.
- Add 2.0 μl of Endo F1 to the reaction. Incubate 1 hour or more at 37°C.
- Monitor cleavage by SDS-PAGE.