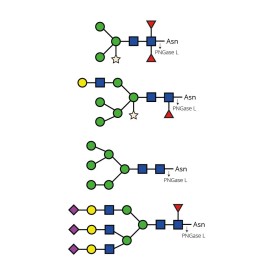
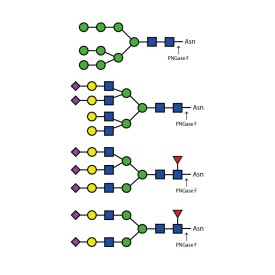
Endoglycosidases
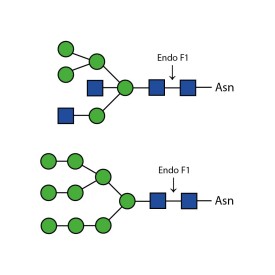
Endoglycosidase F1
E-EF01
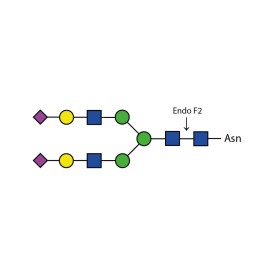
Endoglycosidase F2
E-EF02
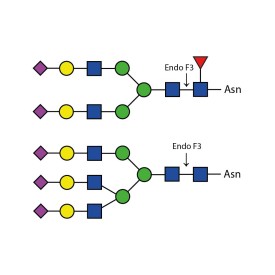
Endoglycosidase F3
E-EF03
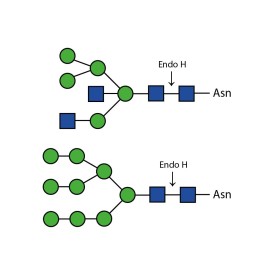
Endoglycosidase H
E-EH02
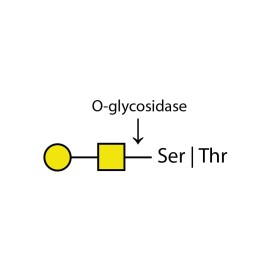
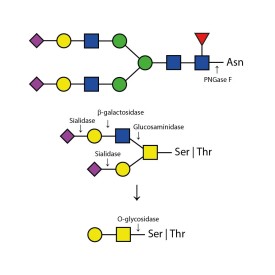
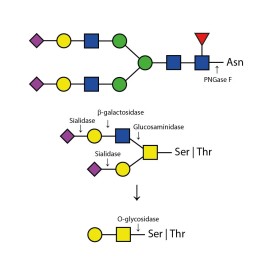
O-glycosidase
E-G001
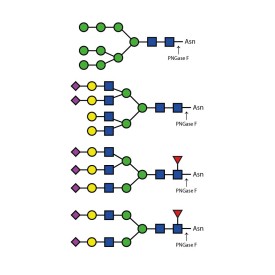
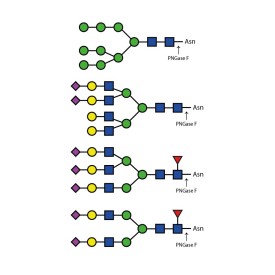
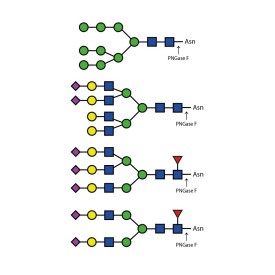
PNGase F (Peptide N Glycosidase F)
E-PNG01-200
Recombinant PNGase F
E-rPNG01
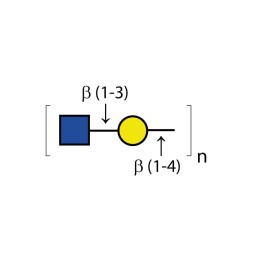
Endo-β-galactosidase
E-XBG01
Enzymatic CarboRelease kit
KE-DG01
Enzymatic DeGlycoMx Kit
KE-DGMX
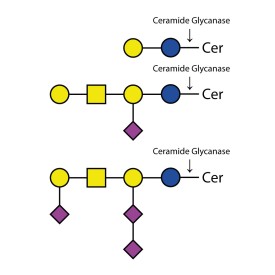
Ceramide Glycanase Kit
LZ-CER-HM-KIT
LudgerZyme PNGase L Kit
LZ-PNGaseL-50-KIT
Recombinant PNGase F
LZ-rPNGaseF-kit
Showing 1 to 14 of 14 (1 Pages)