Monoclonal Antibody Reference Standards
*Formerly sold as SA-MAB4
A mixture of fucosylated, bi-antennary non-sialylated glycan standards, with over 90% purity as assessed by HPLC.
Our new Monoclonal Antibody Reference Standard combines fucosylated N-glycans commonly found on monoclonal antibodies and is a test standard used both in FDA- and EMEA-approved assays worldwide.
Product specifications
Monoclonal Antibody Reference Standards
The use of standards is part of the guidelines listed in the ICH Topic Q6B Specifications: Test Procedures and Acceptance Criteria for biotechnological/Biological Products. They are beneficial for biotherapeutics development, testing of biosimilars and supporting regulatory submissions.
A mixture of fucosylated, bi-antennary non-sialylated glycan standards, with over 90% purity as assessed by HPLC.
Our new Monoclonal Antibody Reference Standard combines fucosylated N-glycans commonly found on monoclonal antibodies and is a test standard used both in FDA- and EMEA-approved assays worldwide.
Specifically, this library can be used as a reference standard for a mixture of glycans commonly found in therapeutic monoclonal antibodies. It can also be implemented as a process control to assess the performance of a glycan labelling protocol and/or a system suitability standard for the analytical platform.
The use of standards is part of the guidelines listed in the ICH Topic Q6B Specifications: Test Procedures and Acceptance Criteria for biotechnological/Biological Products. They are beneficial for biotherapeutics development, testing of biosimilars and supporting regulatory submissions.
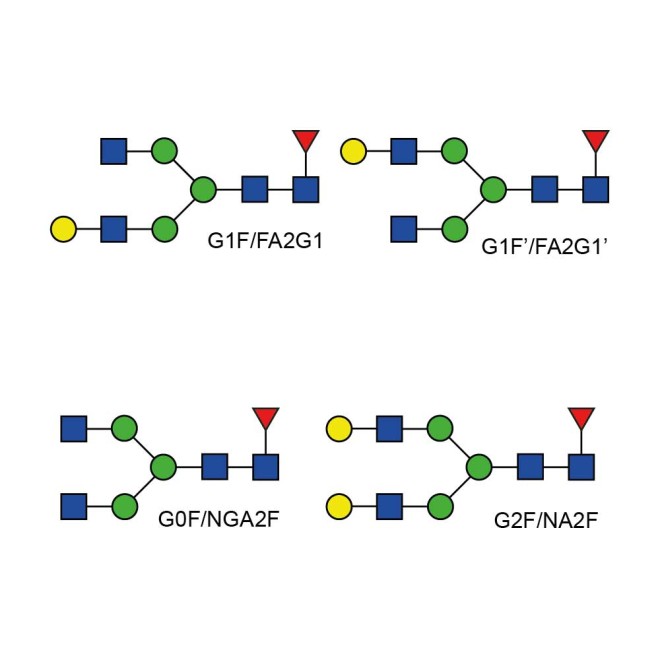
This library combines four N-glycan oligosaccharides which are found on several mammalian glycoproteins including IgG, gamma globulins, and many serum glycoproteins. The glycans are typically purified from the oligosaccharide pool released from porcine thryroglobulin or bovine serum. The glycans are qualified by MS, HPLC and NMR analysis to meet the high purity and quality standards required by advanced analytical techniques. The standard includes the following; G2F(NA2F), G0F(NGA2F), and both G1F(FA2G1) isomers.
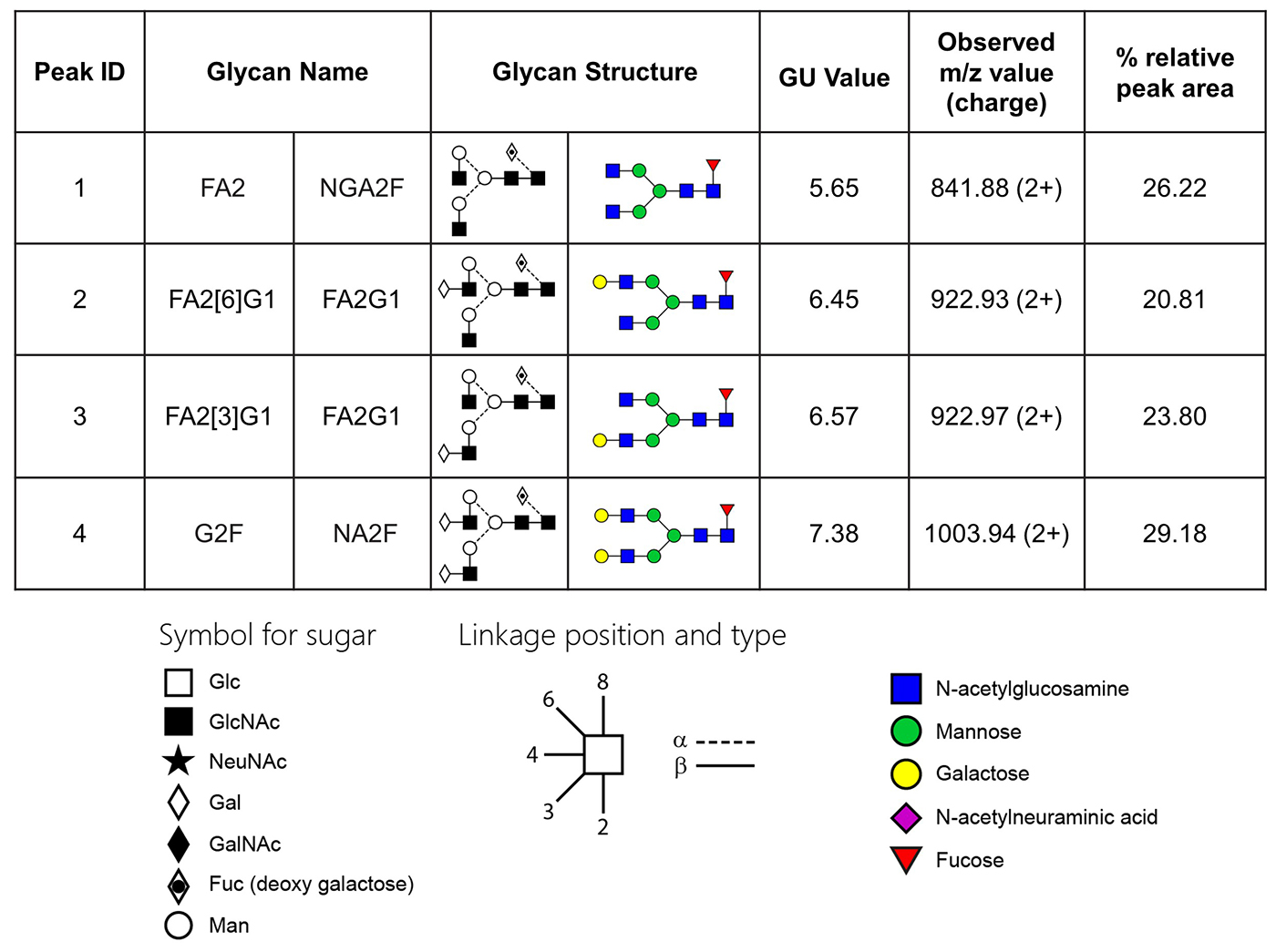
Table 1. Glycan names and structures of each peak from the chromatogram.
Our standards are supported by complete documentation. The certificate of analysis contains the results from the testing used to characterize the material across a complete range of quality characteristics. These standards are well-characterised with documented purity. Each vial contains approximately 10µg of N-glycans.
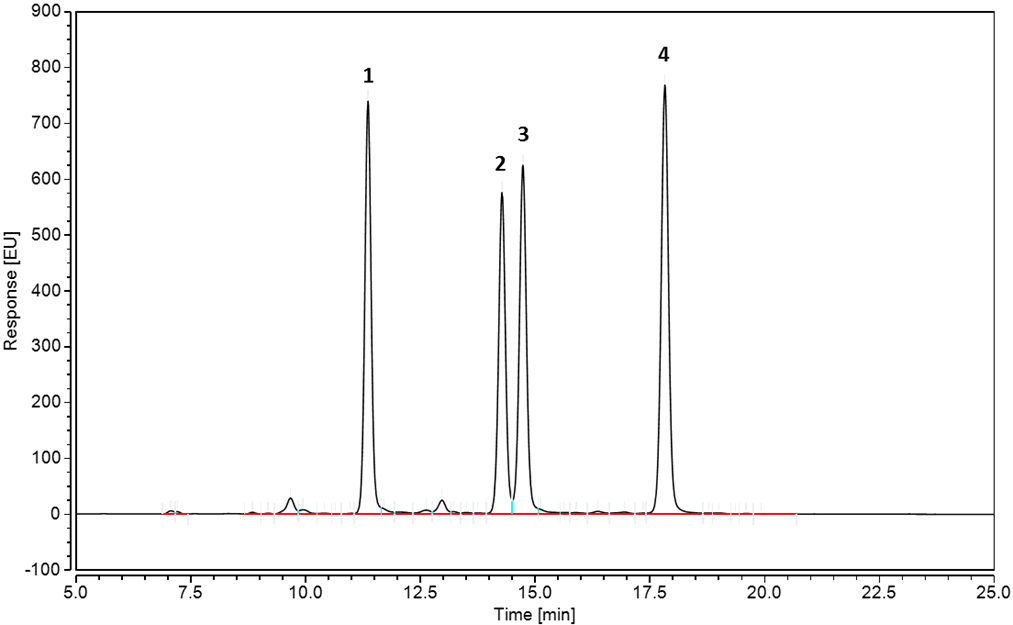
Figure 1. Chromatogram of Procainamide-labelled Mab N-Glycans. Table 1 shows peak assignments.


-660x660.jpg)
 SDS
SDS