Monosaccharide release & labelling kit
References
Abo M, He LP, Sato K, Okubo A. Determination of monosaccharides derivatized with 2-aminobenzoic Acid by capillary electrophoresis. Methods Mol Biol. 984:45-50(2013)
Harazono A1, Kobayashi T, Kawasaki N, Itoh S, Tada M, Hashii N, Ishii A, Arato T, Yanagihara S, Yagi Y, Koga A, Tsuda Y, Kimura M, Sakita M, Kitamura S, Yamaguchi H, Mimura H, Murata Y, Hamazume Y, Sato T, Natsuka S, Kakehi K, Kinoshita M, Watanabe S, Yamaguchi T. A comparative study of monosaccharide composition analysis as a carbohydrate test for biopharmaceuticals. Biologicals May;39(3):171-80. (2011)
He L, Sato K, Abo M, Okubo A, Yamazaki S. Separation of saccharides derivatized with 2-aminobenzoic acid by capillary electrophoresis and their structural consideration by nuclear magnetic resonance. Anal Biochem. Mar 1;314(1):128-34 (2003)
Saddic GN, Dhume ST, Anumula KR. Carbohydrate composition analysis of glycoproteins by HPLC using highly fluorescent anthranilic acid (AA) tag. Methods Mol Biol. 446:215-29(2008)
Sato K, Sato K, Okubo A, Yamazaki S. Determination of monosaccharides derivatized with 2-aminobenzoic acid by capillary electrophoresis. Anal Biochem. Aug 15;251(1):119-21 (1997)
Presentation
Quantitative Monosaccharide Release and 2-AA (2-aminobenzoic acid) Labelling Kit
Monosaccharide analysis is a regulatory requirement laid out in the ICH Q6B guidelines for characterisation of biopharmaceuticals. This information can be used at all stages of drug development as a method of determining the type of glycosylation (N-linked and/or O-linked) and the extent to which glycosylation has occurred. It can also be used to demonstrate consistency between batches for QC lot release during the manufacturing process.
Ludger’s monosaccharide analysis kit is easy to use, reliable and can be used for quantitative routine monosaccharide analysis. (Kit sufficient for 96 analyses).
- Release neutral and amino monosaccharides from your glycoprotein samples by mild acid hydrolysis
- Fluorescent labelling of released monosaccharides with 2-aminobenzoic acid (2AA)
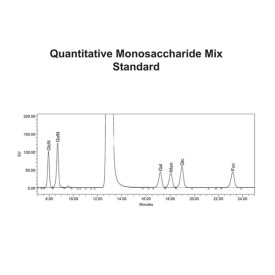
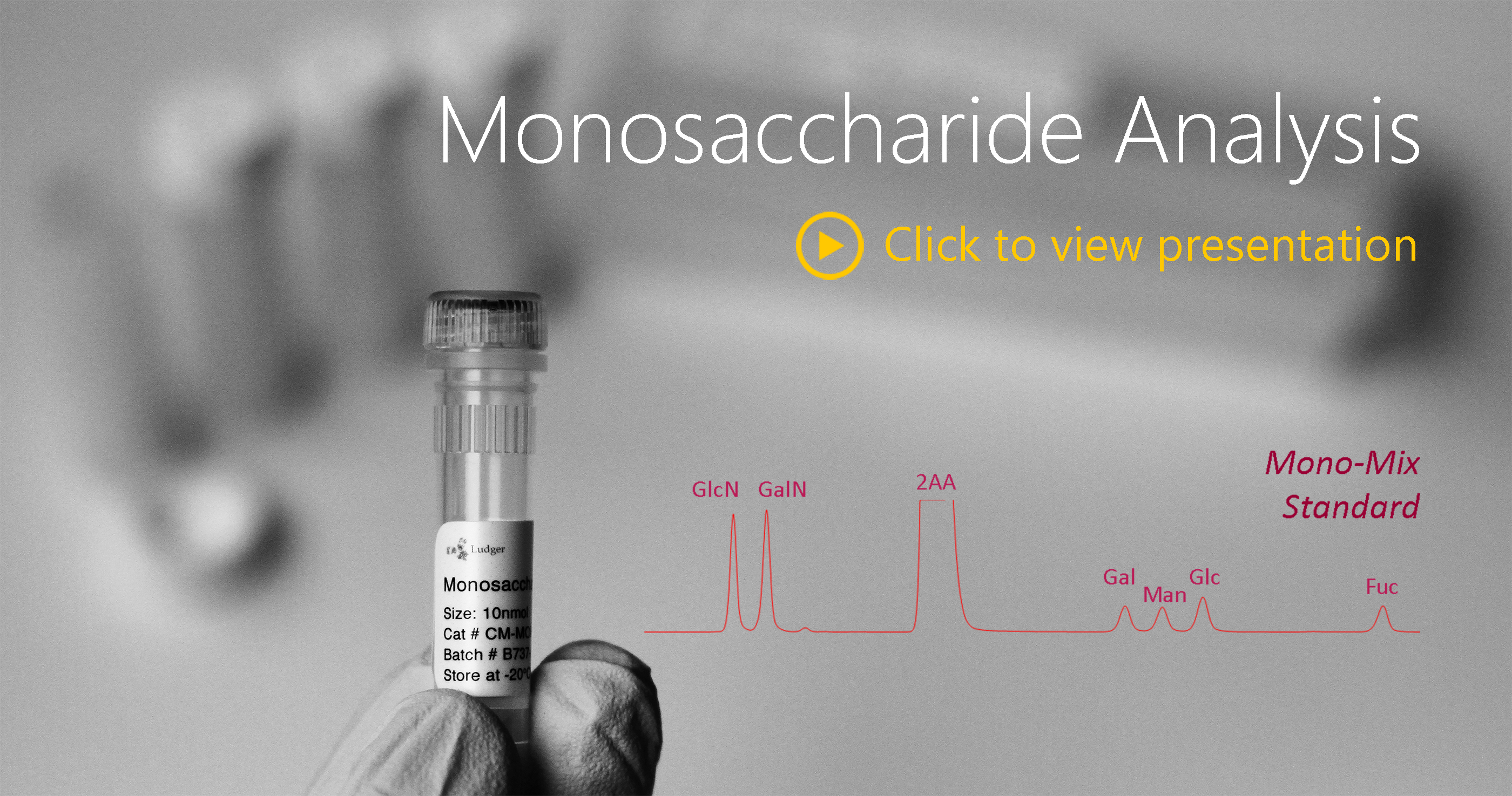
- Relative quantitative analysis of 2AA-labelled monosaccharides compared to standards (GlcN, GalN, Gal, Man, Glc, Fuc, Xyl) by (U)HPLC analysis
-
Info Guides: Monosaccharide Analysis Guide
-
Product Guide: LT-MONO-96
Product/Ordering Information:
Release monosaccharides and label with 2AA:
- LT-MONO-96
- LudgerTag Monosaccharide Release and Labelling Kit
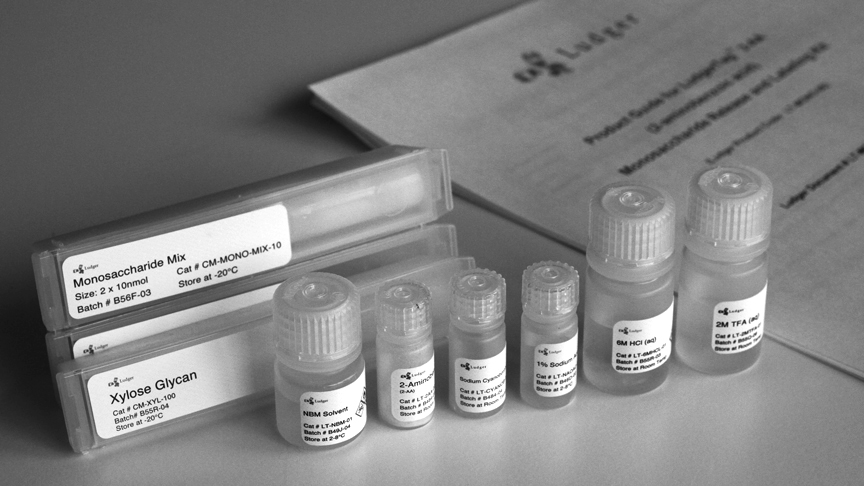
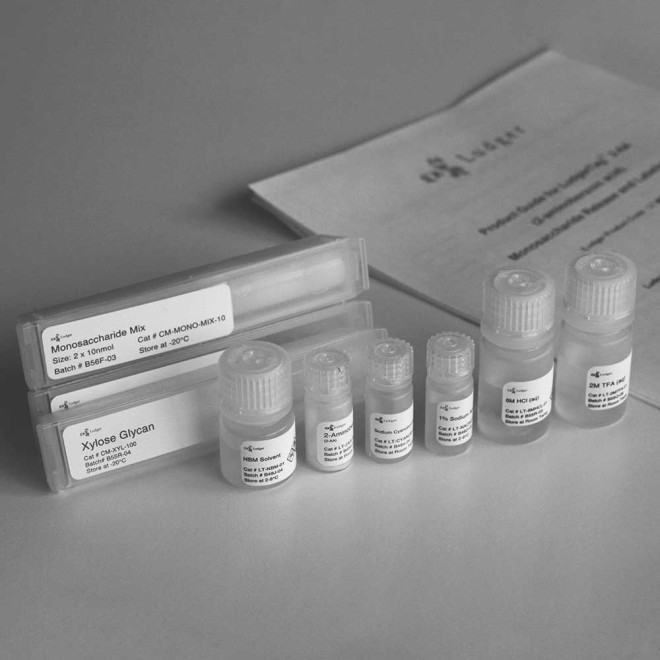
Each kit contains reagents and materials for up to 96 glycoprotein samples, control and standards analysed in parallel or two sets of 48 samples:
- Trifluoroacetic acid (LT-2MTFA-01 - 2 vials)
- Hydrochloric acid (LT-6MHCL-01 - 2 vials)
- Sodium acetate (LT-NAOAC-01 - 2 vials)
- Labelling solvent (LT-NBM-01 - 2 vials)
- 2-aminobenzoic acid (LT-2AA-02 - 2 vials)
- Sodium cyanoborohydride (LT-CYANOB-03 - 2 vials)
- 10 nmols each of glucosamine (GlcN), galactosamine (GalN), 4 Galactose (Gal), glucose (Glc), mannose (Man) and fucose (Fuc) (CM-MONO-MIX-10 - 4 vials)
- 100 nmol of xylose (Xyl) (CM-XYL-100 - 2 vials)
For release and labelling of neutral and amino monosaccharides from glycoprotein therapeutics and pre-released glycans. This kit can be used for full quantitative or routine monosaccharide analysis. Sufficient for 96 samples. The LudgerTag Monosaccharide Analysis Kit contains reagents for the release and fluorescent labelling of monosaccharides found on glycoprotein biopharmaceuticals and standards.
Product specifications
Monosaccharide analysis is a regulatory requirement laid out in the ICH Q6B guidelines for characterisation of biopharmaceuticals. This information can be used at all stages of drug development as a method of determining the type of glycosylation (N-linked and/or O-linked) and the extent to which glycosylation has occurred. It can also be used to demonstrate consistency between batches for QC lot release during the manufacturing process.
One LudgerTag Monosaccharide Analysis Kit contains reagents to release and label up to 96 separate analytical samples per kit, in two sets of reagents (48 samples per set). Typically, 50 micrograms of glycoprotein is analyzed per sample.
The method for monosaccharide analysis is as follows:
1. Release of monosaccharides from the glycoprotein by mild acid hydrolysis.
2. Fluorescent labelling of released monosaccharides with 2-aminobenzoic acid (2AA).
3. Relative quantitative analysis of 2AA-labelled monosaccharides by HPLC or UHPLC
The kit also contains the LudgerTag MonoMix, a monosaccharide standard containing glucosamine, galactosamine, galactose, mannose and fucose. Xylose is included for use as an internal standard.
There are two hydrolysis acids provided. 2 molar trifluoroacetic acid (2M TFA) and 6 molar hydrochloric acid (6M HCl). 2M TFA is good for releasing neutral monosaccharides but is less effective with the N-acetylglucosamine (GlcNAc) and N-acetylgalactosamine (GalNAc) monosaccharides for which we recommend using 6M HCl. We recommend using these acids on separate replicates of your samples.
LudgerTag Release Reagents
Trifluoroacetic acid – 2M TFA
Hydrochloric acid – 6M HCl
LudgerTag Monosaccharide Analysis Quantitative Standards
MonoMix monosaccharide standards – 10 nmols each of glucosamine, galactosamine, galactose, mannose and fucose.
Xylose standard – 100 nmols – used as an internal reference standard for accurate quantification
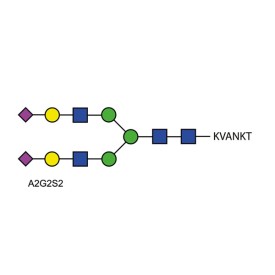
We also recommend a purified glycopeptide standard. Ludger has developed a quantitative standard (BQ-GPEP-A2G2S2-10U) is a complex biantennary N-linked glycan terminating in two N-acetylneuraminic acids. Using this standard will enable you to check the efficiency of glycan release, labeling and recovery and will give you confidence in the accuracy of your monosaccharide measurements.
HPLC – Quantitative Analysis of Labelled Monosaccharides
We offer two choices of column for analysis of the labelled monosaccharides dependent on whether you are using HPLC or UHPLC systems in your laboratory. If using an HPLC, the LudgerSep R2 column (Cat No. LS-R2-4.6×150) gives very good separation of the seven main monosaccharides found in most N-link and O- link glycans. If you have an UHPLC system we recommend using the LudgerSep uR2 column (Cat No. LS-UR2-2.1×50) which can perform an 8 minute separation per sample.
Solvents: The glycan analysis gradients in this guide are based on the following solvents:
Solvent A : BPT solvent – purified water based solvent of 0.2 % butylamine (2 mL per litre), 0.5 % phosphoric acid 5 mL per litre), 1 % tetrahydrofuran (10 mL per litre).
Solvent B : 50 % acetonitrile : 50 % solvent A
We have also tested acetonitrile free solvent systems. Please enquire for further details.


 Product Guide
Product Guide





-275x275.jpg)