Fetuin glycoprotein standard
The Fetuin Glycoprotein Standard was developed for use as a positive control during glycan release and labelling (particularly for DMB sialic acid analysis). Fetuin exists in a variety of glycoforms containing bi-, tri-, and tetraantennary N-linked oligosaccharides with variable sialylation, as well as O-linked glycans.
1 vial containing 250 μg fetuin glycoprotein
Product Specification
The fetuin glycoprotein standard was developed for use during glycan release and labeling(particularly for DMB sialic acid analysis). Fetuin exists in a variety of glycoforms containing bi-, tri-, and tetra-antennary oligosaccharides with variable sialylation, as well as O-linked glycans.
Amount of Glycoprotein Supplied
GCP-FET-05 500 µg
GCP-FET-250U 250 µg
GCP-FET-50Ux4 50 µg per vial – 4 vials
Source Fetal calf serum
Form Dry. Lyophilised powder.
Molecular Weight 36 kDa (protein weight only)
Amount of Protein
GCP-FET-05 300 µg
GCP-FET-250U 150 µg
GCP-FET-50Ux4 32 µg per vial – 4 vials
In comparison to BSA standard, determined by BCA assay. Value rounded to nearest μg.
Analysis
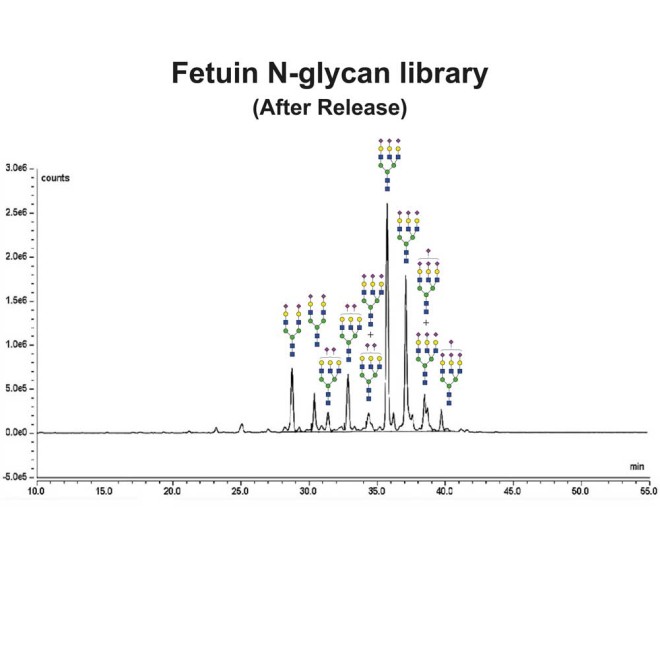
Fetuin glycans were released from the Fetuin Glycoprotein Standard (Cat# GCP-FET-05) using PNGase-F.
Following release the glycans were labeled using 2-Aminobenzamide (2-AB) using the LudgerTag 2-AB Glycan Labeling Kit (Cat# LT-KAB-A2).
Figure 1 shows a LudgerSep N2 HPLC profile of bovine fetuin N-glycans. To thoroughly investigate the N-glycans we first separate them based on charge on a LudgerSep C3 column (Figure 2) and then run each fraction on a LudgerSep N2 column.
Storage Refrigerate (-20°C) both before and after dissolving. This product is stable for at least 5 years as supplied.
Shipping The product is shipped at ambient temperature.
Handling Once dissolved avoid repeated thawing and refreezing, storage over 3 hours at room temperature or above, exposure to light and long term exposure to acid as these will cause glycan desialylation.
Safety This product is non-hazardous and has been purified from natural sources certified to be free of all hazardous material including pathogenic biological agents.