Endo-β-galactosidase
References:
1. Scudder,P., Uemura, K., Doby, J., Fukuda, M.N. & Feizi, T.(1983) Isolation and characterization of an endo-b-galactosidase from Bacteroides fragilis Biochem. J. 213 , 485-494.
2. Scudder,P., Hanfland, Pl, Uemura, K. & Feizi, T. (1984) Endo-b-galactosidases of Bacteroides fragilis and Escherichia freundii hydrolyze linear but not branched oligosaccharide domains of glycolipids of the neolacto series. J. Biol. Chem . 259, 6586-6592.
3. Scudder, P. Tang, P.W., Hounsell, E.F., Lawson, A.M., Mehmet, H. & Feizi, T. (1986) Isolation and characterization of sulfated oligosaccharides released from bovine corneal keratan sulphate by the action of endo-b-galactosidase. Eur. J. Biochem. 157, 365-373.
4. Murata, T., Hattori, T. Amarume, S. Koicki, A. & Usui, T. (2003) Eur. J. Biochem 270, 3709-3719.
5. Hokke, C.H., Bergwerff, A.A., Van Dedem, D.W., Kamerling, J.P, and Vliegenthart, J.F. (1995) Structural analysis of the N- and O-linked carbohydrate chains of recombinant human erythropoietin expressed in Chinese hamster overay cells. Sialylation patters and branch location of dimeric N-acetyllactosamine units. Eur. J. Biochem. 228, 981-1008.
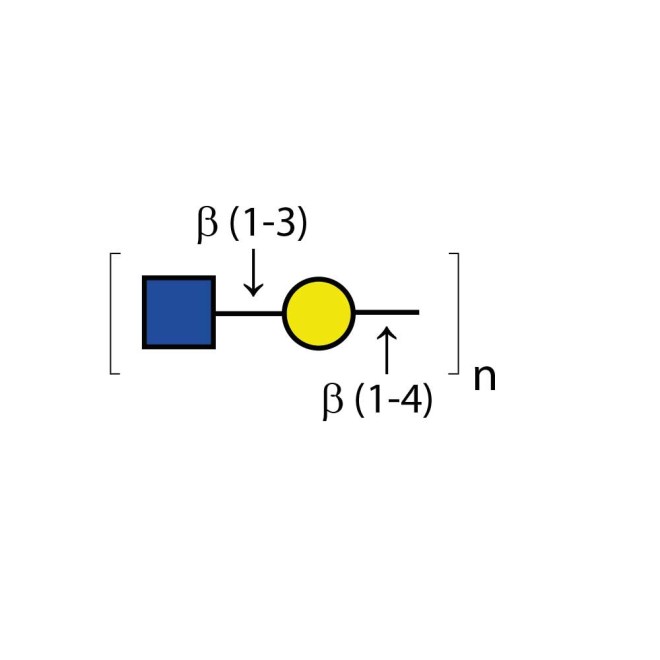
Endo-β-Galactosidase cleaves internal β(1-4) galactose linkages in unbranched, repeating poly-N-acetyllactosamine structures. Sulfated structures such as keratan sulfate are also cleaved. Branching and/or fucosylation of the substrate may decrease or eliminate cleavage.
Endo-β-Galactosidase is useful for identifying and removing poly-N-acetyllactosamine structures on many biologically important glycoconjugates.
Kit includes enzyme plus reaction buffer. Sufficient for up to 60 reactions.
Product Specification
Endo-β-Galactosidase is useful for identifying and removing poly-N-acetyllactosamine structures on many biologically important glycoconjugates.
Source: Recombinant from Bacteroides fragilis in E. Coli
EC: 3.2.1.103
Specificity: Cleaves internal β(1-4) galactose linkages in unbranched, repeating poly-N-acetyllactosamine [GlcNAc-β(1-3)Gal-β(1-4)]n structures are the preferred substrate. Sulfated structures such as keratan sulfate are also cleaved. Branching and/or fucosylation of the substrate may decrease or eliminate cleavage. Sulfation of C-6 on galactose will block cleavage. Oligosaccharidesof the neo-lacto group are cleaved at greatly educed rates depending on the deviation from the preferred substrate. For example, Gal-β(1-3)GlcNAc-β(1-3)Gal-β(1-4)Glc is cleaved at 5×10-5 the rate of keratan sulfate (see ref.4). Specificity is similar to the Escherichia freundii enzyme except that it is limited to cleaving N-acetyllactosamine extensions on tetraantennary structures of erythropoietin (see ref 5).
Contents:
60 µL aliquot of enzyme (0.9 U) in 20 mM tris-HCl, pH 7.5
1 vial reaction buffer- 250mM Sodium phosphate, pH 5.8
Specific Activity: >140 U/mg
Activity: >14 U/mL
Molecular weight: ~32,000 daltons
Optimum pH: 5.8
Suggested usage:
For glycoproteins:
1. Add up to 100 µg of glycoprotein to a tube.
2. Add 4 µL 5X buffer and water to 19 µL.
3. Add 1 µL enzyme.
4. Incubate at 37°C for 2 hrs.
Procedure for oligosaccharides:
Same as above except incubate from several hours to several days depending on the substrate. Add bovine serum albumen to 2 mg/mL to stabilize the protein during extended incubations.
Specific Activity:
One unit of Endo-β-Galactosidase is defined as the amount that will liberate one µmole of reducing sugar per minute at 37°C and pH 5.8 from bovine corneal keratan sulfate.
Stability: Stable at least 24 months when stored properly. Several days exposure to ambient temperature will not reduce activity. Active for at least 5 days under reaction conditions.
Purity: Endo-β-Galactosidase is tested for contaminating protease as follows: 10 µg of denatured BSA is incubated for 24 hours at 37°C with 2 µL of enzyme. SDS-PAGE analysis of the treated BSA shows no evidence of degradation.
The production strain of E. coli has been extensively tested and does not produce any detectable glycosidases.