A2G2S2 quantitative glycopeptide standard
Presentation on: Quantitative Glycopeptide Standard - GPEP-A2G2S2

Quantitative Glycopeptide Standard: BioQuant GPEP-A2G2S2
The quantitative analysis of sialic acids and monosaccharides is a regulatory requirement for any biopharmaceutical drug during the development stage, as well as throughout its whole life cycle (reference: ICH guidelines Q6B and Q5E for comparability studies, EMA monograph on MAb, USP chapters 1084 and 1094 on glycosylation analysis).
At Ludger, we have produced a purified glycopeptide standard which can be used as an internal standard and as a positive control when performing sialic acid quantitation and/or monosaccharide analyses. These methods have been fully validated with this glycopeptide standard as a key element of that process.
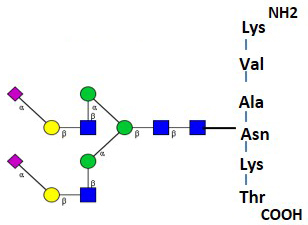
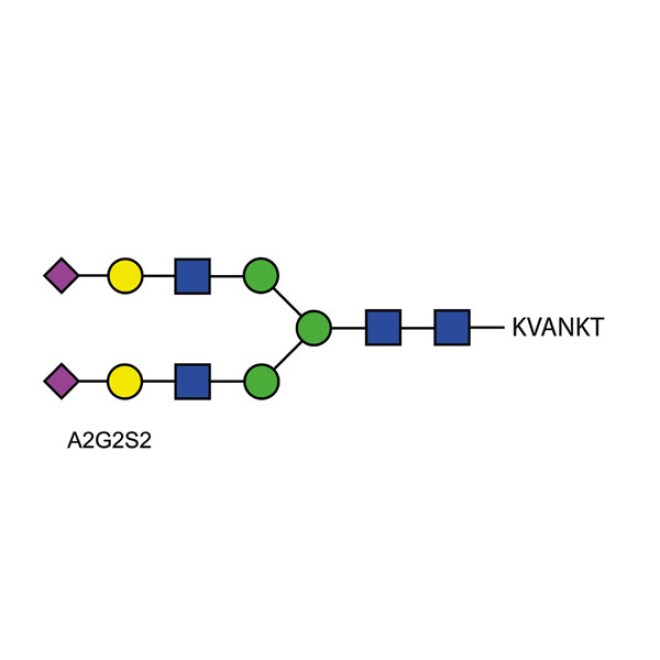
The BioQuant Standard BQ-GPEP-A2G2S2 is a quantitative standard that is a purified N-link glycopeptide comprised of a di-sialylated biantennary glycan of the form A2G2S2. This is attached to the asparagine amino acid of a peptide with the sequence Lysine-Valine-Alanine-Asparagine-Lysine-Threonine (KVANKT).
This standard is our most established BioQuant standard and is widely used in-house and by our customers as a positive control in glycan analysis methods such as:
- Quantitative sialic acid analysis
- Quantitative monosaccharide analysis
- Glycan release and labelling
Contacts
The glycopeptide standard is comprised of an A2G2S2 glycan attached to the asparagine amino acid of a peptide with the sequence Lysine-Valine-Alanine-Asparagine-Lysine-Threonine (KVANKT), 10µg.
m/z: 2865.1763
Quantitative sialic acid or monosaccharide analysis is an important step for developers and manufacturers of biologic drugs. Regulators are putting increasing pressure on companies to perform accurate glycoprofiling on their biopharmaceuticals. These analyses fall within ICH guidelines Q6B and Q5E for comparability studies during product development and after major manufacturing changes. Furthermore, the recent EMA monograph on monoclonal antibodies and forthcoming USP chapters <1084> and <1094> on glycosylation analysis reinforce the need to perform glycoprofiling throughout the drug life cycle.
Ludger Ltd. (www.ludger.com) has produced a purified glycopeptide standard which can be used as an internal standard and positive control when performing sialic acid or monosaccharide analyses in house. This Ludger BioQuant™ Standard (BQ-GPEP-A2G2S2-10U) is a disialylated complex biantennary N-linked glycan containing 2 galactose residues.
Purity of >90% has been assessed by HPLC, correct mass identity assessed by MALDI mass spectrometry. The exact amount of material/concentration is determined by quantitative NMR (qNMR) and quantitative monosaccharide analysis (MA). Quantity values by qNMR and MA agree within 90-110%. MA is traceable to internationally accepted references from USP and dispensed using NIST traceable labware. qNMR is traceable to a NIST SRM traceable CRM analysed to the ISO 17025 standard. A detailed certificate of analysis is given for each standard. This contains comprehensive documentation, lot-specific values, expiration date and storage information.
Product Specification
Quantitative Glycopeptide Standard: BioQuant GPEP-A2G2S2
This standard is our most established BioQuant standard and is widely used in-house and by our customers as a positive control in glycan analysis methods such as:
- Quantitative sialic acid analysis
- Quantitative monosaccharide analysis
- Glycan release and labelling
This will enable you to check the efficiency of glycan release, labeling, and recovery.
Confidence in your sialic acid or monosaccharide measurements
The BioQuant GPEP-A2G2S2 glycopeptide standard gives you confidence in the accuracy of your results.
The glycopeptide is comprised of an A2G2S2 glycan attached to the asparagine amino acid of a peptide with the sequence Lysine-Valine-Alanine-Asparagine-Lysine-Threonine (KVANKT).
System Suitability Use to demonstrate efficiency of labelling, column efficiency and repeatability of glycoanalysis
Regulatory Submissions Supports drugs regulatory submission by demonstrating consistent and reproducible results
Well Characterised Sialylated Glycopeptide Standard Identity and purity is assessed by HPLC, NMR and Mass Spectrometry techniques
Quality Assurance Monosaccharide and Sialic acid analysis is traceable to internationally accepted references from USP and dispensed using NIST traceable labware
Use as a Positive Control The GPEP-A2G2S2 standard can run in parallel with your sialic acid release or monosaccharide analysis
Quantitative sialic acid or monosaccharide analysis is an important step for developers and manufacturers of biologic drugs. Regulators are putting increasing pressure on companies to perform accurate glycoprofiling on their biopharmaceuticals. These analyses fall within ICH guidelines Q6B and Q5E for comparability studies during product development and after major manufacturing changes. Furthermore, the recent EMA monograph on monoclonal antibodies and forthcoming USP chapters <1084> and <1094> on glycosylation analysis reinforce the need to perform glycoprofiling throughout the drug life cycle.
Ludger Ltd. has produced a purified glycopeptide standard which can be used as an internal standard and positive control when performing sialic acid or monosaccharide analyses in house. The Ludger BioQuant Standard is a glycopeptide standard comprised of an N-linked sialyated glycan (A2G2S2 – alternative names: A2, G0 glycan) attached to the asparagine amino acid of a valine-alanine-asparagine- lysine-threonine (KVANKT) peptide
Purity of >90% has been assessed by HPLC, correct mass identity assessed by MALDI mass spectrometry. The exact amount of material/concentration is determined by quantitative NMR (qNMR) and quantitative monosaccharide analysis (MA). Quantity values by qNMR and MA agree within 90-110%. MA is traceable to internationally accepted references from USP and dispensed using NIST traceable labware. qNMR is traceable to a NIST SRM traceable CRM analysed to the ISO 17025 standard. A detailed certificate of analysis is given for each standard. This contains comprehensive documentation, lot-specific values, expiration date and storage information.


-660x660.jpg)